In the realm of molecular science, the ability to observe chemical bonds forming and breaking in real-time has long been a holy grail. Recent breakthroughs in femtosecond X-ray laser technology have brought this dream closer to reality, enabling researchers to capture the intricate dance of atoms with unprecedented clarity. This cutting-edge technique, often referred to as the "single-molecule movie camera," is revolutionizing our understanding of chemical reactions at their most fundamental level.
The concept hinges on the use of ultra-short X-ray pulses, each lasting mere femtoseconds (quadrillionths of a second). These bursts of light are brief enough to freeze the motion of atoms mid-reaction, allowing scientists to construct detailed frame-by-frame sequences of molecular transformations. Unlike conventional methods that average out behavior across countless molecules, this approach captures the behavior of individual molecules, providing insights that were previously unimaginable.
What makes this technology truly groundbreaking is its ability to probe the very essence of chemical bonds. When atoms interact to form molecules, the process occurs on timescales far too rapid for traditional observation techniques. Femtosecond X-ray lasers overcome this limitation by delivering pulses so short that they can effectively photograph the positions of atoms before significant movement occurs. The resulting "molecular movies" reveal not just static structures, but the dynamic evolution of chemical systems.
The implications for chemistry and related fields are profound. For decades, scientists have relied on theoretical models to predict how reactions proceed. While these models have been remarkably successful, direct experimental verification at the atomic scale has remained elusive. With femtosecond X-ray lasers, researchers can now test these theories against actual observations, potentially leading to revisions in our fundamental understanding of chemical kinetics and reaction mechanisms.
One particularly exciting application lies in the study of enzyme catalysis. Enzymes are nature's molecular machines, accelerating biochemical reactions by factors of millions. How they achieve this remarkable feat at the atomic level has been a subject of intense debate. The single-molecule movie approach allows scientists to watch enzymes in action, tracking the subtle rearrangements of atoms that enable their extraordinary catalytic power. Such insights could inspire the design of artificial catalysts with similar efficiency.
The technology also promises to transform materials science. Many important material properties - from conductivity to strength - depend on how atoms are arranged and bonded. By observing these bonds form and break under different conditions, researchers can engineer materials with precisely tailored characteristics. This capability could lead to breakthroughs in everything from energy storage to quantum computing.
Developing this capability required overcoming significant technical challenges. Creating X-ray pulses of sufficient intensity and brevity demanded advances in laser physics and accelerator technology. Similarly, detecting the faint signals from single molecules necessitated the development of extremely sensitive detectors. Perhaps most crucially, researchers had to devise methods to precisely synchronize the X-ray pulses with the initiation of chemical reactions.
The experimental setup typically involves initiating reactions with optical laser pulses, then probing them with precisely timed X-ray pulses. This pump-probe technique, repeated with varying delays between the pulses, builds up a sequence of snapshots that can be assembled into a coherent movie of the reaction. The approach has been successfully applied to systems ranging from simple diatomic molecules to complex biomolecules.
Looking ahead, scientists anticipate even more dramatic advances as the technology matures. Future developments may allow for higher resolution imaging, capturing not just atomic positions but the movement of electrons during reactions. This could provide unprecedented insights into electron transfer processes that underpin phenomena from photosynthesis to battery operation. Additionally, combining these techniques with advanced computational methods promises to create a powerful feedback loop between theory and experiment.
While the technology is still in its relative infancy, early results have already challenged some long-held assumptions about chemical reactivity. Certain reactions appear to proceed through intermediate states that were previously undetectable, while others follow pathways quite different from theoretical predictions. As more systems are studied, we may need to revise our basic understanding of how molecules interact and transform.
The societal implications of this research are potentially enormous. From developing more efficient solar cells to designing new pharmaceuticals, the ability to observe and understand chemical processes at this fundamental level could accelerate innovation across numerous fields. In drug design, for instance, watching how potential medications interact with their targets at the atomic scale could streamline the development process and lead to more effective treatments.
As with any transformative technology, challenges remain. The facilities required for these experiments are currently large and expensive, limiting widespread access. Researchers are working to develop more compact and affordable systems, but significant engineering hurdles persist. Additionally, interpreting the complex data generated by these experiments requires sophisticated computational tools and theoretical frameworks.
Despite these challenges, the future looks bright for single-molecule movie technology. As the methods continue to improve and become more accessible, we can expect a flood of new discoveries about the molecular world. What was once the realm of theory and indirect inference is now becoming directly observable, opening new frontiers in our understanding of matter and its transformations.
The development of femtosecond X-ray lasers for chemical imaging represents a remarkable convergence of physics, chemistry, and engineering. It stands as a testament to human ingenuity and our relentless pursuit of knowledge at the smallest scales. As this technology continues to evolve, it promises to reveal the hidden choreography of atoms and molecules, fundamentally changing how we understand and manipulate the molecular building blocks of our world.

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025
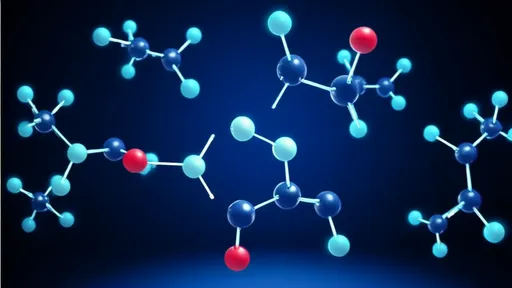
By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025